Electrodialysis (0D)
Introduction
Electrodialysis, an electrochemical separation technology, has been utilized to desalinate water for decades. Compared with other technologies, such as reverse osmosis (RO), electrodialysis shows advantages in desalinating brackish waters with moderate salinity by its less intense energy consumption, higher water recovery, and robust tolerance for adverse non-ionic components (e.g., silica and biological substances) in water. Between the electrodes of an electrodialysis stack (a reactor module), multiple anion- and cation-exchange membranes are alternately positioned and separated by spacers to form individual cells. When operated, electrodialysis converts electrical current to ion flux and, assisted by the opposite ion selectivity of cation- and anion-exchange membranes (cem and aem), moves ion from one cell to its adjacent cell in a cell-pair treatment unit (Figure 1). The ion-departing cell is called a diluate channel and the ion-entering cell a concentrate channel. Recovered (desalinated) water is collected from diluate channles of all cell pairs while the concentrate product can be disposed of as brine or retreated. More overview of the electrodialysis technology can be found in the References.
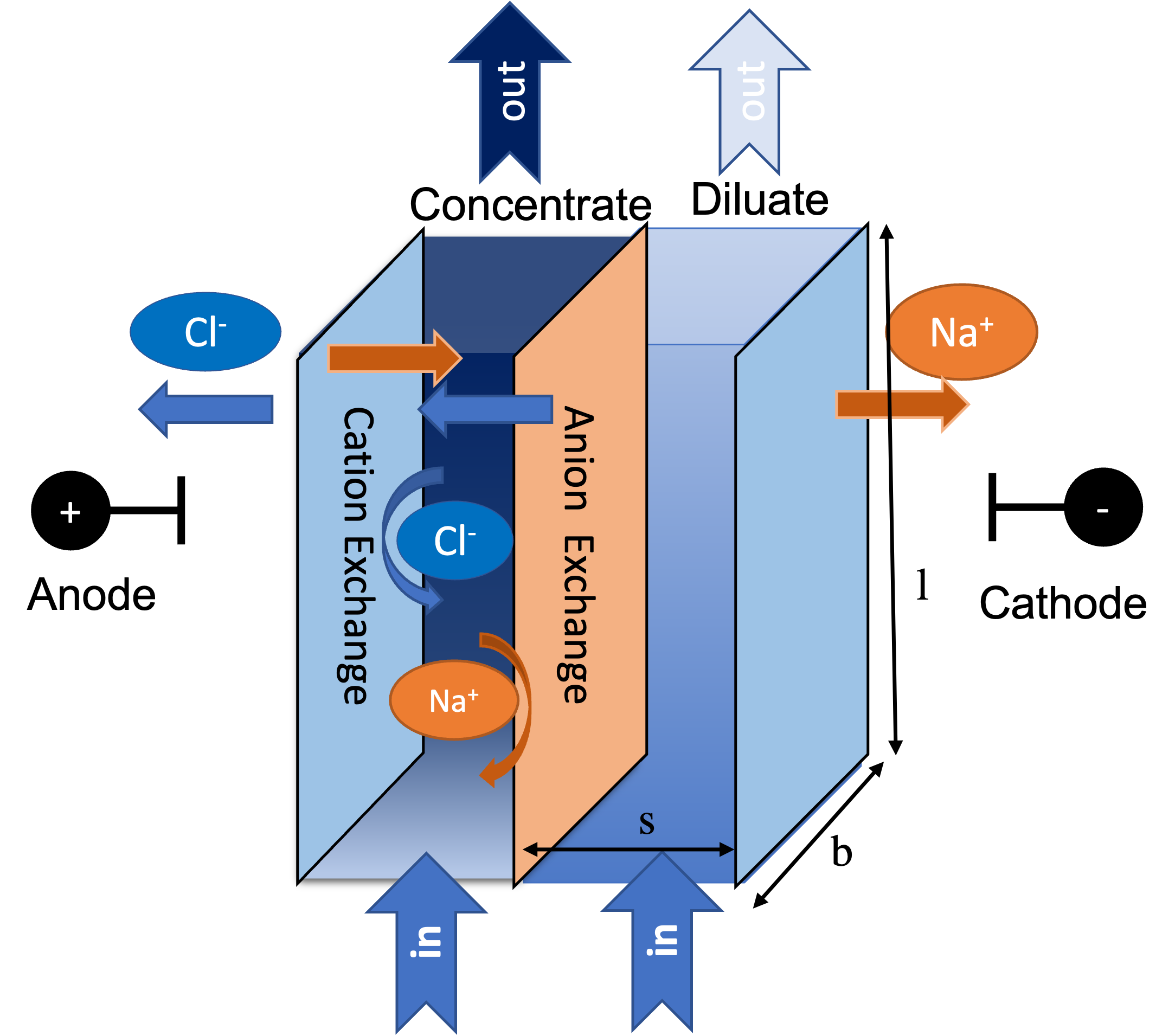
Figure 1. Schematic representation of an electrodialysis cell pair
One cell pair in an electrodialysis stack can thus be treated as a modeling unit that can multiply to larger-scale systems. The presented electrodialysis model establishes mathematical descriptions of ion and water transport in a cell pair and expands to simulate a stack with a specified cell-pair number. Modeled mass transfer mechanisms include electrical migration and diffusion of ions and osmosis and electroosmosis of water. The following key assumptions are based on.
The concentrate and diluate channels have identical geometry.
For each channel, component fluxes are uniform in the bulk solutions (the 0-dimensional assumption) and are set as the average of inlet and outlet of each channel.
Steady state: all variables are independent on time.
Co-current flow operation.
Electrical current is operated below the limiting current.
Ideality assumptions: activity, osmotic, and van’t Hoff coefficients are set at one.
All ion-exchange membrane properties (ion and water transport number, resistance, permeability) are constant.
Detailed concentration gradient effect at membrane-water interfaces is neglected.
Constant pressure and temperature through each channel.
Control Volumes
This model has two control volumes for the concentrate and diluate channels.
Diluate_channel
Concentrate_channel
Ports
On the two control volumes, this model provides four ports (Pyomo notation in parenthesis):
inlet_diluate (inlet)
outlet_diluate (outlet)
inlet_concentrate (inlet)
outlet_concentrate (outlet)
Sets
This model can simulate the electrodialysis desalination of a water solution containing multiple species (neutral or ionic). All solution components ( H2O, neutral solutes, and ions) form a Pyomo set in the model. For a clear model demonstration, this document uses a NaCl water solution as an instance hereafter. The user can nevertheless expand the component set as needed to represent other feed water conditions.
Description |
Symbol |
Indices |
|---|---|---|
Time |
\(t\) |
[t] ([0])1 |
Phase |
\(p\) |
[‘Liq’] |
Component |
\(j\) |
[‘H2O’, ‘Na_+’, ‘Cl_-‘] |
Ion |
\(j\) |
[‘Na_+’, ‘Cl_-’] 2 |
Membrane |
n/a |
[‘cem’, ‘aem’] |
- Notes
1 The time set index is set as [0] in this steady-state model and is reserved majorly for the future extension to a dynamic model.
2 “Ion” is a subset of “Component” and uses the same symbol j.
Degrees of Freedom
Applying this model to a NaCl solution yields 33 degrees of freedom (Table 2), among which temperature, pressure, and component molar flow rate are state variables that are fixed as initial conditions. The rest are parameters that should be provided in order to fully solve the model.
Description |
Symbol |
Variable Name |
Index |
Units |
DOF Number 1 |
|---|---|---|---|---|---|
Temperature, inlet_diluate |
\(T^D\) |
temperature |
None |
\(K\) |
1 |
Temperature, inlet_concentrate |
\(T^C\) |
temperature |
None |
\(K\) |
1 |
Pressure, inlet_diluate |
\(p^D\) |
temperature |
None |
\(Pa\) |
1 |
Pressure, inlet_concentrate |
\(p^C\) |
temperature |
None |
\(Pa\) |
1 |
Component molar flow rate, inlet_diluate |
\(N_{j, in}^D\) |
flow_mol_phase_comp |
[t], [‘Liq’], [‘H2O’, ‘Na_+’, ‘Cl_-‘] |
\(mol s^{-1}\) |
3 |
Component molar flow rate, inlet_concentrate |
\(N_{j, in}^C\) |
flow_mol_phase_comp |
[t], [‘Liq’], [‘H2O’, ‘Na_+’, ‘Cl_-‘] |
\(mol s^{-1}\) |
3 |
Water transport number |
\(t_w\) |
water_trans_number_membrane |
[‘cem’, ‘aem’] |
dimensionless |
2 |
Water permeability |
\(L\) |
water_permeability_membrane |
[‘cem’, ‘aem’] |
\(m^{-1}s^{-1}Pa^{-1}\) |
2 |
Voltage or Current 2 |
\(U\) or \(I\) |
voltage or current |
[t] |
\(\text{V}\) or \(A\) |
1 |
Electrode areal resistance |
\(r_{el}\) |
electrodes_resistance |
[t] |
\(\Omega m^2\) |
1 |
Cell pair number |
\(n\) |
cell_pair_num |
None |
dimensionless |
1 |
Current utilization coefficient |
\(\xi\) |
current_utilization |
None |
dimensionless |
1 |
Spacer thickness |
\(s\) |
spacer_thickness |
none |
\(m\) |
1 |
Membrane areal resistance |
\(r\) |
membrane_surface_resistance |
[‘cem’, ‘aem’] |
\(\Omega m^2\) |
2 |
Cell width |
\(b\) |
cell_width |
None |
\(\text{m}\) |
1 |
Cell length |
\(l\) |
cell_length |
None |
\(\text{m}\) |
1 |
Thickness of ion exchange membranes |
\(\delta\) |
membrane_thickness |
[‘cem’, ‘aem’] |
\(m\) |
2 |
diffusivity of solute in the membrane phase |
\(D\) |
solute_diffusivity_membrane |
[‘cem’, ‘aem’], [‘Na_+’, ‘Cl_-‘] |
\(m^2 s^{-1}\) |
4 |
transport number of ions in the membrane phase |
\(t_j\) |
ion_trans_number_membrane |
[‘cem’, ‘aem’], [‘Na_+’, ‘Cl_-‘] |
dimensionless |
4 |
- Note
1 DOF number takes account of the indices of the corresponding parameter.
2 A user should provide either current or voltage as the electrical input, in correspondence to the “Constant_Current” or “Constant_Voltage” treatment mode (configured in this model). The user also should provide an electrical magnitude that ensures a operational current below the limiting current of the feed solution.
Solution component information
To fully construct solution properties, users need to provide basic component information of the feed solution to use this model, including identity of all solute species (i.e., Na +, and Cl - for a NaCl solution), molecular weight of all component species (i.e., H2O, Na +, and Cl -), and charge and electrical mobility of all ionic species (i.e., Na +, and Cl -). This can be provided as a solution dictionary in the following format (instanced by a NaCl solution).
ion_dict = {
"solute_list": ["Na_+", "Cl_-"],
"mw_data": {"H2O": 18e-3, "Na_+": 23e-3, "Cl_-": 35.5e-3},
"electrical_mobility_data": {"Na_+": 5.19e-8, "Cl_-": 7.92e-8},
"charge": {"Na_+": 1, "Cl_-": -1},
}
This model, by default, uses H2O as the solvent of the feed solution.
Information regarding the property package this unit model relies on can be found here:
Equations
This model solves mass balances of all solution components (H2O, Na +, and Cl - for a NaCl solution) on two control volumes (concentrate and diluate channels). Mass balance equations are summarized in Table 3. Mass transfer mechanisms take account of solute electrical migration and diffusion and water osmosis and electroosmosis. Theoretical principles, e.g., continuity equation, Fick’s law, and Ohm’s law, to simulate these processes are well developed and some good summaries for the electrodialysis scenario can be found in the References.
Description |
Equation |
Index set |
|---|---|---|
Component mass balance |
\(N_{j, in}^{C\: or\: D}-N_{j, out}^{C\: or\: D}+J_j^{C\: or\: D} bl=0\) |
\(j \in \left['H_2 O', '{Na^{+}} ', '{Cl^{-}} '\right]\) |
mass transfer flux, concentrate, solute |
\(J_j^{C} = \left(t_j^{cem}-t_j^{aem} \right)\frac{\xi I}{((bl) z_j F}-\left(\frac{D_j^{cem}}{\delta ^{cem}} +\frac{D_j^{aem}}{\delta ^{aem}}\right)\left(c_j^C-c_j^D \right)\) |
\(j \in \left['{Na^{+}} ', '{Cl^{-}} '\right]\) |
mass transfer flux, diluate, solute |
\(J_j^{D} = -\left(t_j^{cem}-t_j^{aem} \right)\frac{\xi I}{((bl) z_j F}+\left(\frac{D_j^{cem}}{\delta ^{cem}} +\frac{D_j^{aem}}{\delta ^{aem}}\right)\left(c_j^C-c_j^D \right)\) |
\(j \in \left['{Na^{+}} ', '{Cl^{-}} '\right]\) |
mass transfer flux, concentrate, H2O |
\(J_j^{C} = \left(t_w^{cem}+t_w^{aem} \right)\left(\frac{I}{(bl)F}\right)+\left(L^{cem}+L^{aem} \right)\left(p_{osm}^C-p_{osm}^D \right)\left(\frac{\rho_w}{M_w}\right)\) |
\(j \in \left['H_2 O'\right]\) |
mass transfer flux, diluate, H2O |
\(J_j^{D} = -\left(t_w^{cem}+t_w^{aem} \right)\left(\frac{I}{(bl)F}\right)-\left(L^{cem}+L^{aem} \right)\left(p_{osm}^C-p_{osm}^D \right)\left(\frac{\rho_w}{M_w}\right)\) |
\(j \in \left['H_2 O'\right]\) |
Additionally, several other equations are built to describe the electrochemical principles and electrodialysis performance.
Description |
Equation |
|---|---|
Ohm’s Law |
\(U = \frac{I r_{tot}}{bl}\) |
Resistance calculation |
\(r_{tot}=n\left(r^{cem}+r^{aem}+\frac{s}{\kappa^C}+\frac{s}{\kappa^D}\right)+r_{el}\) |
Electrical power consumption |
\(P=UI\) |
Water-production-specific power consumption |
\(P_Q=\frac{UI}{3.6\times 10^6 nQ_{out}^D}\) |
Overall current efficiency |
\(I\eta=\sum_{j \in[cation]}{\left[\left(N_{j,in}^D-N_{j,out}^D\right)z_j F\right]}\) |
All equations are coded as “constraints” (Pyomo). Isothermal and isobaric conditions apply.
Nomenclature
Symbol |
Description |
Unit |
|---|---|---|
Parameters |
||
\(\rho_w\) |
Mass density of water |
\(kg\ m^{-3}\) |
\(M_w\) |
Molecular weight of water |
\(kg\ mol^{-1}\) |
Variables and Parameters |
||
\(N\) |
Molar flow rate of a component |
\(mol\ s^{-1}\) |
\(J\) |
Molar flux of a component |
\(mol\ m^{-2}s^{-1}\) |
\(b\) |
Cell/membrane width |
\(m\) |
\(l\) |
Cell/membrane length |
\(m\) |
\(t\) |
Ion transport number |
dimensionless |
\(I\) |
Current |
\(A\) |
\(U\) |
Voltage over a stack |
\(V\) |
\(n\) |
Cell pair number |
dimensionless |
\(\xi\) |
Current utilization coefficient (including ion diffusion and water electroosmosis) |
dimensionless |
\(z\) |
Ion charge |
dimensionless |
\(F\) |
Faraday constant |
\(C\ mol^{-1}\) |
\(D\) |
Ion Diffusivity |
\(m^2 s^{-1}\) |
\(\delta\) |
Membrane thickness |
\(m\) |
\(c\) |
Solute concentration |
\(mol\ m^{-3}\) |
\(t_w\) |
Water electroosmotic transport number |
dimensionless |
\(L\) |
Water permeability (osmosis) |
\(ms^{-1}Pa^{-1}\) |
\(p_{osm}\) |
Osmotic pressure |
\(Pa\) |
\(r_{tot}\) |
Total areal resistance |
\(\Omega m^2\) |
\(r\) |
Membrane areal resistance |
\(\Omega m^2\) |
\(r_{el}\) |
Electrode areal resistance |
\(\Omega m^2\) |
\(s\) |
Spacer thickness |
\(m\) |
\(\kappa\) |
Solution conductivity |
\(S m^{-1}\ or\ \Omega^{-1} m^{-1}\) |
\(\eta\) |
Current efficiency for desalination |
dimensionless |
\(P\) |
Power consumption |
\(W\) |
\(P_Q\) |
Specific power consumption |
\(kW\ h\ m^{-3}\) |
\(Q\) |
Volume flow rate |
\(m^3s^{-1}\) |
Subscripts and superscripts |
||
\(C\) |
Concentrate channel |
|
\(D\) |
Diluate channel |
|
\(j\) |
Component index |
|
\(in\) |
Inlet |
|
\(out\) |
Outlet |
|
\(cem\) |
Cation exchange membrane |
|
\(aem\) |
Anion exchange membrane |
References
Strathmann, H. (2010). Electrodialysis, a mature technology with a multitude of new applications. Desalination, 264(3), 268-288.
Strathmann, H. (2004). Ion-exchange membrane separation processes. Elsevier. Ch. 4.
Campione, A., Cipollina, A., Bogle, I. D. L., Gurreri, L., Tamburini, A., Tedesco, M., & Micale, G. (2019). A hierarchical model for novel schemes of electrodialysis desalination. Desalination, 465, 79-93.